
SL Paper 1
Which solution forms when phosphorus(V) oxide, P4O10, reacts with water?
Markscheme
C
Examiners report
Which property increases down Group 1, the alkali metals?
A. Atomic radius
B. Electronegativity
C. First ionization energy
D. Melting point
Markscheme
A
Examiners report
Which trend is correct, going down group 1?
A. Melting point increases
B. Reactivity decreases
C. First ionisation energy increases
D. Electronegativity decreases
Markscheme
D
Examiners report
Which describes an atom of bismuth, Bi (Z = 83)?
Markscheme
C
Examiners report
A well answered question regarding finding principal energy level and valence electrons from position on the periodic table.
Which is a d-block element?
A. Ca
B. Cf
C. C
D. Co
Markscheme
D
Examiners report
Which of the following would have the same numerical value for all elements in the same period?
A. Highest energy levels occupied
B. Energy sub-levels occupied
C. Orbitals occupied
D. Valence electrons
Markscheme
A
Examiners report
A challenging question with a high discrimination index. 46 % of the candidates chose the correct answer A (the highest energy levels occupied have the same numerical value for all elements in the same period). A high proportion of candidates chose C (orbitals occupied have the same value ….) and a significant proportion chose B (energy sub-levels occupied have the same value …).
Which species has the same electron configuration as argon?
A. Br−
B. Ca2+
C. Al3+
D. Si4+
Markscheme
B
Examiners report
Which oxide will dissolve in water to give the solution with the lowest pH?
A.
B.
C.
D.
Markscheme
A
Examiners report
Fairly well answered. Almost 70% of candidates could identify that non-metallic oxides would dissolve in water to yield a substance of low pH.
Which is an f-block element?
A. Sc
B. Sm
C. Sn
D. Sr
Markscheme
B
Examiners report
Which element is in the p-block?
A. Pb
B. Pm
C. Pt
D. Pu
Markscheme
A
Examiners report
Which metal has the strongest metallic bond?
A. Li
B. Na
C. K
D. Rb
Markscheme
A
Examiners report
Three elements, X, Y, and Z are in the same period of the periodic table. The relative sizes of their atoms are represented by the diagram.
Which general trends are correct?
Markscheme
C
Examiners report
The question involved the identification of three trends. 43% of the candidates selected the correct trends in ionization energy, effective nuclear charge and acidity of the oxides. The most commonly chosen distractor reversed the trend in the acidity of the oxides.
Which oxide, when added to water, produces the solution with the highest pH?
A. Na2O
B. SO3
C. MgO
D. CO2
Markscheme
A
Examiners report
Which trends are correct across period 3 (from Na to Cl)?
I. Atomic radius decreases
II. Melting point increases
III. First ionization energy increases
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which equation represents the first electron affinity of chlorine?
A. Cl(g)+e-→ Cl-(g)
B. Cl2(g) + e- → Cl-(g)
C. Cl+(g) + e- → Cl(g)
D. Cl(g) → Cl+(g) + e-
Markscheme
A
Examiners report
Which property shows a general increase from left to right across period 2, Li to F?
A. Melting point
B. Electronegativity
C. Ionic radius
D. Electrical conductivity
Markscheme
B
Examiners report
Which element is a lanthanide?
A. Hf
B. Tb
C. U
D. Y
Markscheme
B
Examiners report
Which of the following shows a general increase across period 3 from to ?
A. Ionic radius
B. Atomic radius
C. Ionization energy
D. Melting point
Markscheme
C
Examiners report
Although 75% of candidates had the correct answer, this question about trends across period 3 was not a very good discriminator.
Which describes the oxide of sodium, Na2O?
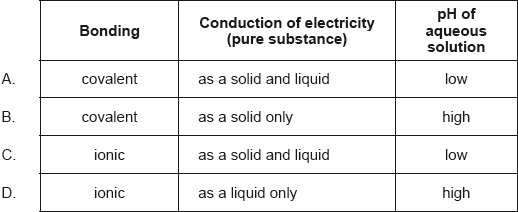
Markscheme
D
Examiners report
Which combination describes the acid–base nature of aluminium and phosphorus oxides?
Markscheme
A
Examiners report
Which species will require the least energy for the removal of one electron?
A. Na+
B. Mg+
C. Al2+
D. C3+
Markscheme
B
Examiners report
Which gases are acidic?
I. nitrogen dioxide
II. carbon dioxide
III. sulfur dioxide
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
What are typical characteristics of metals?
Markscheme
A
Examiners report
Very disappointing results with a large percentage of the candidates thinking metals have a high electron affinity.
Which of the following is the electron configuration of a metallic element?
A. [Ne] 3s2 3p2
B. [Ne] 3s2 3p4
C. [Ne] 3s2 3p6 3d3 4s2
D. [Ne] 3s2 3p6 3d10 4s2 4p5
Markscheme
C
Examiners report
Which oxide dissolves in water to give a solution with a pH below 7?
A. MgO
B. Li2O
C. CaO
D. P4O10
Markscheme
D
Examiners report
Which property increases down group 1?
A. atomic radius
B. electronegativity
C. first ionization energy
D. melting point
Markscheme
A
Examiners report
Which statement is correct?
A. Atomic radius decreases down group 17.
B. First ionization energy decreases down group 1.
C. Atomic radius increases across period 3 from Na to Cl.
D. First ionization energy decreases across period 3 from Na to Cl.
Markscheme
B
Examiners report
How do the following properties change down Group 17 of the periodic table?
Markscheme
C
Examiners report
74 % of the candidates chose the correct trends for ionization energy and ionic radius down Group 17.
Which oxides produce an acidic solution when added to water?
I. Al2O3 and SiO2
II. P4O6 and P4O10
III. NO2 and SO2
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Which element has the highest metallic character in Group 14?
A. C
B. Si
C. Ge
D. Sn
Markscheme
D
Examiners report
Which element is found in the 4th group, 6th period of the periodic table?
A. Selenium
B. Lead
C. Chromium
D. Hafnium
Markscheme
D
Examiners report
A very well answered question. 76% of the candidates were able to identify the element in the fourth group and sixth period of the periodic table as hafnium. The most commonly chosen distractor was lead which is in group 14 not 4.
Which increase across a period from left to right?
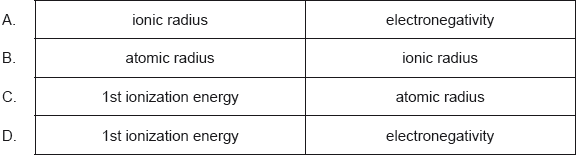
Markscheme
D